I am a fan of small amine-containing compounds with relatively short history in synthetic organic chemistry. Such molecules are admittedly hard to come by, but when I see them, I marvel at what might be done with them and why people have not considered them more broadly.
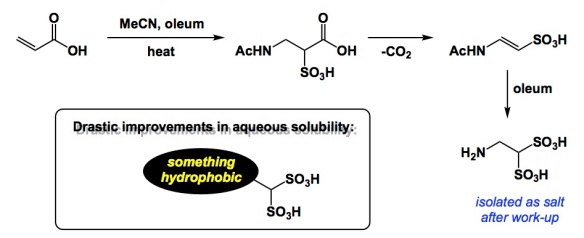
The other day I was flipping through the 2017 Strem catalog for no logical reason other than I got this shiny new booklet in the mail and felt guilty to toss it straight into the blue recycling bin, the destination of all catalogs I receive on a weekly basis. My attention got piqued by 2-aminoethane-1,1-disulfonic acid (let’s call it ADSA), which is offered by Strem for some unknown reason (metal catalysts is their main focus). Unaware of ADSA’s existence, I looked through standard search engines and found very little prior to 2010. There was some work done by Wagner and co-workers in the 60’s, but not much since. The synthesis of this compound is simple, yet interesting as it involves a modified Ritter reaction with oleum, decarboxylation, and sulfonation of the enamide. ADSA offers as an outstanding way to improve aqueous solubility of fairly hydrophobic molecules such as Alexa Fluor dyes. I find the geminal bis(sulfonate) functionality rather interesting because it reminds me of bis(phosphonates), which are of course miles ahead in terms of demonstrated use and significance as components of drugs that prevent the loss of bone mass.
http://www.sciencedirect.com/science/article/pii/S0040403910006908

it would be interesting to add some KNO3 to oleum, to see if we could nitrate instead of sulfonation. (H2N)2C=C(NO2)2 known as FOX-7 is a ultra-low-sensitivity high brisance explosive but its preparation from 4,6-dihydroxy-2-methylpyrimidine is pretty expensive, and unpleasant
This is an interesting thought, but might bring the wrong folks to this page! 😉
by the way, I don’t believe the reaction mechanism is quite correct in the scheme above, since it presumes both oxidation and reduction in the last two steps. If the last step (= reduction) was due to SO2 addition, the regiochemistry would be different (1,2-disulfonic instead of 1,1-disulfonic acid). Since this reaction mix would be quite a hard-to-analyze black fuming porridge from which the final product crystallizes out, it is reasonable to assume the intermediates were not identified by rigorous analysis but rather guessed.
I think far more likely mechanism is second gem sulfonation of the sulfonated Ritter reaction intermediate 1, followed by decarboxylation (and later acetamide hydrolysis). I like this better because it would proceed without any redox changes.
(Not that I haven’t seen examples like this, for example Vilsmeier-Haack on bromoacetic acid seems to produce regular decarboxylated product bromomalondialdehyde at first but after the third formylation in one pot the bromine is reductively lost and the final product is triformylmethane. But this involves only one reduction, not oxidation-reduction in one pot)
You might have a point there. I was merely stating what I saw and yes, it is a really weird proposal…