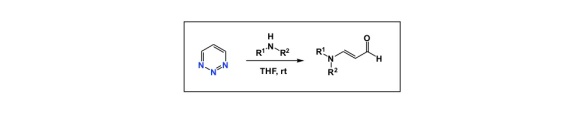
In part due to my long-standing interest in heterocycle-driven drug discovery, I was kind of surprised to see this Org. Lett. paper. In this article, Boger and colleagues showcase a fascinating new way of making vinylogous formamides from 1,2,3-triazines. What is curious here is the very fact that triazines can participate in nucleophilic addition reactions. I have seen many attempts to introduce these rings into bioactive substances, but now that triazines have been shown to be excellent electrophiles toward amines, I should adjust my expectations for this class of molecules. In the Boger report, the reactivity of the parent 1,2,3-triazine was exemplified using secondary amines. The preparative sequence is straightforward: mix amine and triazine in THF at room temperature, and off you go. The C4 position is the preferred point of attack, leading to the extrusion of nitrogen gas. Clearly, the preferred delocalization of the negative charge is behind the documented regiochemistry. Orbital considerations are also consistent with this mode of reactivity. On balance, this is a nice method to make vinylogous formamides. It also suggests to never use 1,2,3-triazines as constituents of bioactive structures.