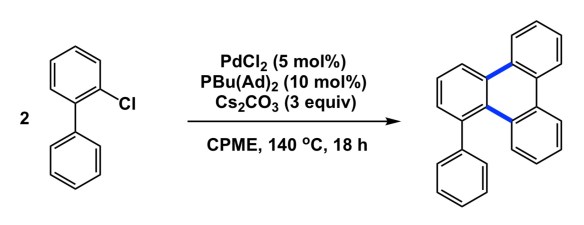
I have to get back to blogging, partly because I miss it and partly because RSC mentioned that I had a blog when they ran an announcement related to my new position as an Associate Editor of Chemical Science (http://blogs.rsc.org/sc/2018/12/20/meetandreiyudinchemicalscienceassociateeditor/?doing_wp_cron=1547228932.9969739913940429687500). Some people are wondering what has been going on, why I am not posting anything. So I guess this is as good a time as ever to get back to writing. Fittingly, we had Prof. Kei Murakami of Nagoya University visit us yesterday as part of his Canadian tour. Kei gave a great talk, and I want to focus on the paper he published not long ago (http://science.sciencemag.org/content/sci/359/6374/435.full.pdf). Apart from seeing an amazingly facile route to nanoribbons, I marvel at the simplicity of the reaction below that had enabled the rapid assembly of the graphene-like building blocks. I wonder why is it that this process has taken so long to reveal itself. Just look at what is happening here and never mind the mechanism, for which we do not have time. My question is how many times in the past, likely all over the world, students must have run a coupling between a biphenyl chloride and some amine, or a boronic acid, or what not. I know that homocoupling is a common by-product of palladium-catalyzed processes, but are you trying to tell me that no one ever ran control experiments in his/her mechanistic studies and did not notice the (homocoupling – 2) mass? Nope, which is why we now have a new reaction. Kei left for Queen’s University after his talk here, and I wish him all the best for what should be a notable career!

It’s nice to see you blogging again and congratulations on making associate editor!
Thanks! Good to be back.