Here’s a synthetic post, dedicated to the talented folks in total synthesis who have a lot of really cool tricks up their sleeves. I am going to talk about just one (or two) steps in Tohru Fukuyama’s fairly recent lyconadine synthesis. The sequence goes through one of my favorite processes – a microscopic reverse of an electrocyclization… I mentioned a similar reaction in the past when I referred to our own work in electrocyclic ring-opening of bromoaziridines. Take a look at a clever use of electrocyclization (its microscopic reverse, that is) in efforts to create a complex 7-memebred ring. First of all, please note the dibromocyclopropane preparation. Wait – before we go any further: do you know whose process this is? If you are thinking of Prof. Makosza from Poland, you are correct as he is the man! I already commented on his vicarious aromatic substitution mechanism. The phase transfer-catalyzed dibromocyclopropanation hails from his lab as well. The yield here is not great, presumably since we are dealing with a fairly challenging substrate… Then comes the key step, which is carried out in pyridine at reflux. A ton of fun, no doubt, but the result is impressive – the ring system is set up and the benzyl group is gone… There is an elimination pathway that competes, but these are minor details. It is still an elegant sequence. I think one nice lesson here is to always remember the principle of microscopic reversibility, which is not simple when thinking about retrosynthesis, in my view!
Monthly Archives: October 2013
Good old condensation reactions
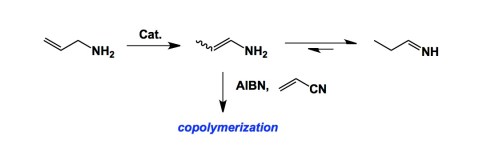
We have finished our grant proposal and managed to submit it on time. As I mentioned yesterday, this grant deals with the chemistry of boron-containing heterocycles and their biological properties as serine protease inhibitors. A preliminary account of our borocycle chemistry driven by boryl isocyanides, appeared earlier this summer (see my July 21 post). Besides what I think is an interesting structure-driven means to optimizing the cellular permeability and activity of these molecules, we have an approach to place boron in heterocycles using simple condensation reactions. As I was thinking about condensation chemistry, I recalled to mind some of my favorite papers from the past. A lot has been said about enamines in recent years, and for a good reason. Originally developed by Stork, enamines are the engine of many innovative synthetic approaches, including organocatalysis. Yet, if you think about the parent “NH2” enamine, it has remained a curiosity due to its highly unstable nature. Back in 2001, Novak and colleagues published a thought-provoking paper that trapped these species in a radical-mediated polymerization. This publication has always been one of my all-time favorite papers. The way to generate the parent enamine shown below is not through condensation (can’t really use thermodynamically controlled reactions). Instead, the authors used transition metal-catalyzed isomerization. Afterwards, they cleverly co-polymerized the enamine under radical conditions before it had a chance to undergo tautomerization. To me, this is super cool.
 http://pubs.acs.org/doi/pdf/10.1021/ja011609i
http://pubs.acs.org/doi/pdf/10.1021/ja011609i
P.S. I am sure my lab might notice the wording “simple enamines” in the paper title…
The APS 2015 website is now live
Right now I am in the middle of grant writing with my student Adam Zajdlik… The deadline is tomorrow and we are trying to get the last remaining items in order. This is a joint grant with Dr. Aaron Schimmer of the Princess Margaret Hospital here in Toronto. Our idea is to use boron-containing heterocycles as proteasome inhibitors. Adam has become quite a pro at making them. Plus we now have Victoria Corless in our team, which will provide further momentum to the effort. Due to this deadline I am not going to post my usual scientific blurb, but I am going to give you what I promised on Friday… Please bookmark the website that has a bit more information about the American Peptide Conference in 2015:

