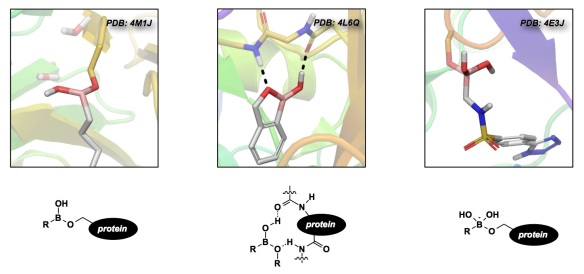
My lab and I have been heavily entrenched in the design of boron-containing covalent inhibitors of proteases. In my view, synthetic students optimally relate to the challenges of chemical biology when they think about the fundamentals of polar bimolecular reactivity. This is exactly what I like to teach in my classes when I describe enzymes as giant nucleophiles. If you then take a look at the electrophilic options out there, you might first consider epoxides, aziridines, and acrylates. These molecules are useful, but are somewhat boring because they offer a singular outcome upon interaction with the enzyme target. This is not the case with boron, which is something my PhD student Diego Diaz and I realized and presented in a recent Nature Chemistry paper. We have analyzed well-known covalent inhibitors containing boron and concluded that this element is unique in its ‘chameleonic’ ability to display a range of coordination modes upon interaction with protein targets. It turns out that organoboron inhibitors leverage boron’s capacity to switch between an uncharged trigonal-planar structure to an anionic tetrahedral one. It is here where boron deviates from common electrophiles that display a singular type of interaction with active site nucleophiles. As a corollary, boron is well suited to act as a flexible anchoring element that is adaptive to structural changes upon binding. Where do you think we are taking this? If you have guessed that we are using these properties to adjust the residence time of boron-containing inhibitors, you are on the right track.
https://www.nature.com/nchem/journal/v9/n8/full/nchem.2814.html
