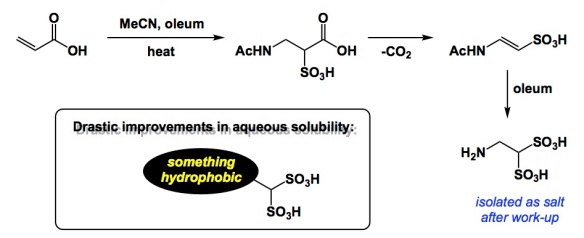
I typically do not comment on deprotection conditions, but there is something special in the two papers below. When I read the one by Lattanzi and colleagues, I thought that their nice asymmetric chemistry had been somewhat overshadowed by a single carbamate cleavage condition using TBAF. I am not sure how many of you are experienced with aziridines, but they do not easily withstand typical Boc removal with TFA. When I saw the yield of 98%, I was literally floored. In the interest of full disclosure, I learned about this deprotection from a paper I recently refereed. I dug a bit deeper and found that the TBAF condition goes back to the 2004 Tetrahedron report by Coudert and colleagues. In it, the authors considered a number of substrates and even carried out mechanistic studies that seem to suggest that the reaction proceeds through the formation of carbamoyl fluoride. Really strange, I know, but there is some very clever evidence in the Coudert paper. That 2004 study centered on the use of common amines and there was nothing as exotic as aziridines. Unless you are familiar with the pain of dealing with these three-membered rings, you might not think that a new way to remove Boc is worthy of note. This simple TBAF trick might be consequential to a lot of people interested in the chemistry of aziridines.
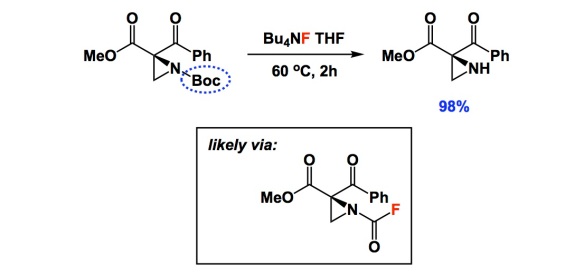
Lattanzi: http://pubs.acs.org/doi/abs/10.1021/ol3017066
Coudert: http://www.sciencedirect.com/science/article/pii/S0040402004012487