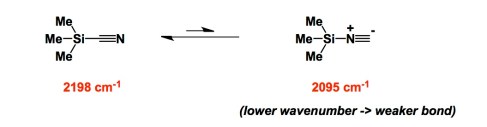
Here is a heterocycle we do not hear a whole lot about these days: azete. Once you wonder about this kind of molecule, you will find yourself at the outskirts of organic chemistry! Azetes do not get mentioned at all when we teach sp2-rich nitrogen heterocycles (such as pyridine and pyrrole). However, they do exist and are isolable, despite being antiaromatic. I am always reminded of Alan Marchand’s instructive remark that one should not incorporate thermodynamically controlled steps when building strained molecules. Accordingly, azetes are made by a thermal decomposition of the cyclopropenyl azide (see below). The very existence of azetes is clearly kinetic in origin, which is to say that these four-membered rings can be obtained once a sufficient barrier preventing their decomposition has been secured. In the language of those brave souls who consider making molecules of this kind, successful isolation of an azete can be achieved once several tert-butyl groups are placed at the periphery of the molecule. The NMR of tris(tert-butyl)azete is the most interesting part of the classic Angewandte paper by Regitz (link below). There are only two groups of signals corresponding to tert-butyl groups, which means that the molecule is a time-averaged form of the two structures shown in the magenta rectangle below. It is as if the atoms are dancing around and the molecule keeps distorting itself…
http://onlinelibrary.wiley.com/doi/10.1002/anie.198608421/abstract
Why am I bringing up this obscure heterocycle? As I teach my CHM 249 class, I always contemplate really weird things. I feel a need to mention this sort of stuff as it makes me feel particularly good to be a synthetic chemist. What can be better? We create our own subject, the stuff that often has no business being stable, let alone found in nature. Just think about it: have you ever heard of synthetic astronomy? Nope. But synthetic chemistry – by all means! Synthetic biology seems to be emerging, though.