There are many different ways of coming up with research ideas in organic synthesis. Broadly, chemists tend to be driven by either functional significance of their favorite molecules or by methods of synthesis. Some of us simply desire to reach a given target and then move on to the next one, without necessarily spending any extra time deciphering how molecules function. These are the three main types of research directions in organic synthesis.
I want to talk about a very frequently encountered and, judging by the amount of papers, fertile area of inquiry: studying the effect of solvent on reactivity. I cannot say that this domain of knowledge ever attracted me, although my lab does have a list of our favourite solvents that dictate our preferences for running reactions. However, I would never seek this to be a defining feature of a research project. It just isn’t my thing. But whatever rocks your boats, ladies and gentlemen! On the subject of solvent systems, many people feel passionate about all sorts of reaction milieu. Ionic liquids, fluorous media, biphasic systems based on water/organic solvents, you name it. Some of this research is purely Edisonian, but every now and then I see papers that go into my “skeletons from the closet” folder. This file grows from year to year and occupies a segment of my computer I am almost afraid to visit. I know each of these papers documents intriguing results, but I am just not yet prepared to fully understand the corresponding rationale.
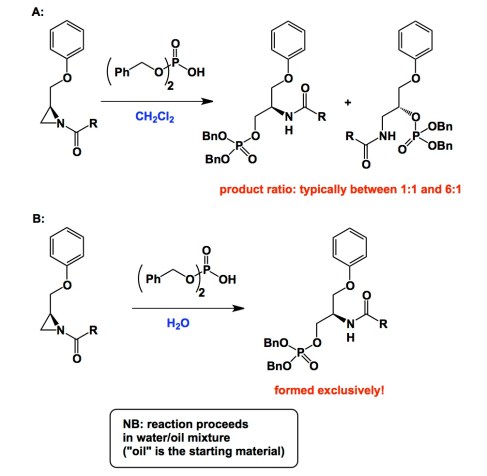
Below is a typical example. It comes courtesy of Professor Zwanenburg. On a recent trip to the Netherlands I met several of Zwanenburg’s disciples who spoke very fondly of his contributions over the years. I am certainly one of Zwanenburg’s fans. Take a look at the graphic and a link below. The facts described suggest that nucleophilic ring-opening of the acyl aziridine ring (inset A) is not selective in dichloromethane, but improves to 100% in selectivity in water (inset B). There are examples of solvent-driven regioselectivity out there, but I am not aware of cases (and I urge you, reader, to draw my attention to prominent examples I am missing), in which a profound improvement in selectivity was recorded upon switching to a biphasic system. Of note is the rationale put forth by the authors of the Chem. Comm. paper. They suggest that the methylene portion of the aziridine is more “exposed” to the aqueous system when the reaction is carried out in the biphasic regime. Apparently, this accounts for the relative accessibility of the methylene group compared to the methane portion of the ring. Many years have passed since this Chem. Comm. paper appeared and I am still puzzled…
http://pubs.rsc.org/en/content/articlelanding/2001/cc/b009294k#!divAbstract