Of late, I have been having a number of discussions about pi-systems with my students. There are many reasons for this surge of interest, too many to mention in one post. This discussion of pushing the boundaries of unusual pi-reactivity reminded me of some really innovative work coming out of Prof. Neil Garg’s lab at UCLA. Neil has pioneered some cool ways of making (and using) indolynes, the distant cousins of benzynes. One of his earlier papers related to this technology appeared back in 2010. I really appreciate this work, which is due to my lab’s long-standing interest in medium sized rings, particularly that of indolactam V, a well-known PKC inhibitor. In Neil’s indolactam synthesis, indolyne intermediates are put to clever use. What you see below is the key step that enables this synthesis. The chemistry involves regioselective addition of an amine nucleophile to the strained indolyne pi-system.
Category Archives: Uncategorized
Counting oxidation states
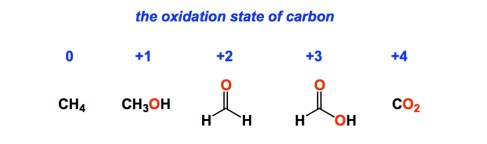
I want to talk about the concept of an oxidation state. According to IUPAC, it is “a measure of a degree of oxidation of an atom in a substance”. While inorganic chemistry has a fairly clear-cut set of rules about how to count oxidation states of metals in organometallic complexes, it is certainly not so simple in the case of organic compounds. There are vastly different ways of assigning oxidation states to carbon centres in organic molecules and things get unnecessarily confusing. I decided to take a look at some online resources… In the following video (http://www.youtube.com/watch?v=M4Q7Ba1ELIQ) the oxidation state of carbon in methane is assigned to -4, while in ethane it is assigned to -3! Of course, everyone understands that this is sheer nonsense. If we follow these guidelines, an aldehyde and a ketone will have DIFFERENT oxidation states at the carbonyl centre, which is not consistent with their fairly similar properties.
I would say that we have to go back to the drawing board and ask a question: why bother with oxidation states at all? There is only one purpose: to understand structure and reactivity better. In inorganic chemistry, Pd in the oxidation state “0” does things that Pd in the oxidation state “+2” is not known for (or known to do differently). This knowledge really helps us. Why don’t we keep things simpler in organic chemistry? Well, hybridization states and C-C bonds (including multiple ones!) screw us up. To make things more useful in this oxidation state mess, I think it is important to first consider one-carbon molecules. I will just look at the following four and unambiguously assign oxidation states based on the number of carbon-heteroatom bonds.
 More elaborate carbonaceous molecules are composed of fragments that correspond to the variations of the blocks above. I admit that carbon-carbon multiple bonds make counting tricky. If a carbon atom has one pi bond and no heteroatom partners, its oxidation state is “+1” (e.g. ethylene). If a carbon is BOTH part of a pi-system and is connected to a heteroatom, the oxidation level is defined as the number of pi-bonds plus the number of heteroatoms attached to the carbon under consideration. Take the following case as an example:
More elaborate carbonaceous molecules are composed of fragments that correspond to the variations of the blocks above. I admit that carbon-carbon multiple bonds make counting tricky. If a carbon atom has one pi bond and no heteroatom partners, its oxidation state is “+1” (e.g. ethylene). If a carbon is BOTH part of a pi-system and is connected to a heteroatom, the oxidation level is defined as the number of pi-bonds plus the number of heteroatoms attached to the carbon under consideration. Take the following case as an example:
The reason I really like things this way is that they enable me to think about heterocycles with some clarity. If someone knows of a better way to easily count oxidation states, let me know.
Unleashing some powerful reactivity
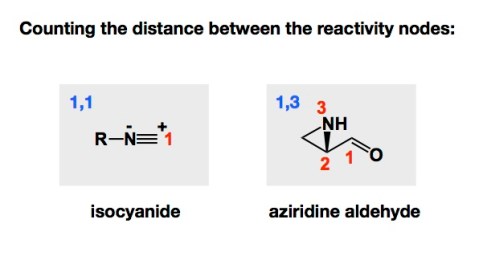
The Passerini and Ugi processes are two of the best-known multicomponent reactions that are based on the isocyanide functional group. In each of these processes, the isocyanide reacts with two carbonyl components. A carboxylic acid and an aldehyde are engaged in the Passerini process, while in the Ugi reaction it is the imine that reacts with the isocyanide instead of an aldehyde. When we started looking at amphoteric aziridine aldehydes with Ryan Hili back in 2006, we thought of aziridine aldehydes as 1,3-molecules based on the distance between the nucleophilic and electrophilic nodes of reactivity. This was a nice way to differentiate from isocyanides.
While aziridine aldehyde reactivity was in its infancy back in 2006, the isocyanide’s innate ability to react with the donor (eg aldehyde) and acceptor (eg acid) components had ample precedent in the Ugi and Passerini reactions I just talked about. Ryan and I felt that something must happen if you simply mix an aziridine aldehyde and an isocyanide. However, this simplest mode of reactivity has been elusive thus far. While we had plenty of luck with other processes, it has been kind of frustrating not to be able to find a precedent for what appears logical: mix isocyanide and aziridine aldehyde and get something cool. We are still trying to reduce this idea to practice. Unless my students correct me, aziridine aldehydes and isocyanides just stare at each other in solution. I still don’t understand why. Most likely Mother Nature is trying to teach me a lesson in kinetics here…
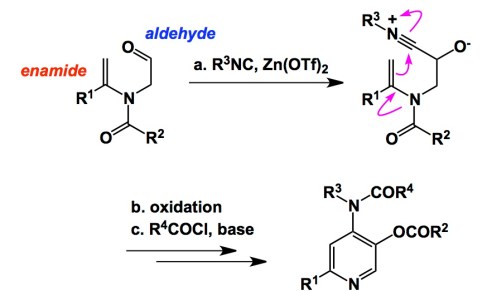
Along these lines, here is an interesting example that I really enjoyed reading about. The chemistry comes courtesy of Prof. Wang in Tsinghua University, China, and details the chemistry of enamide aldehydes that use their enamide portion in order to attack the isonitrilium ion, which is in turn created during the well-established isocyanide attack at the aldehyde (just take a page from the Passerini reaction!). The elements of amphoteric reactivity are on full display here. As a result of this well-orchestrated sequence, one gets access to polysubstituted pyridines. In the graphic below I am not attempting to illustrate all the gory details. While a couple of steps (oxidation and acylation) are left behind, the core of the process is present. Enamido aldehydes seem to be more than adequate to unleash the synthetic prowess of the isocyanide functionality…
Lab revelations
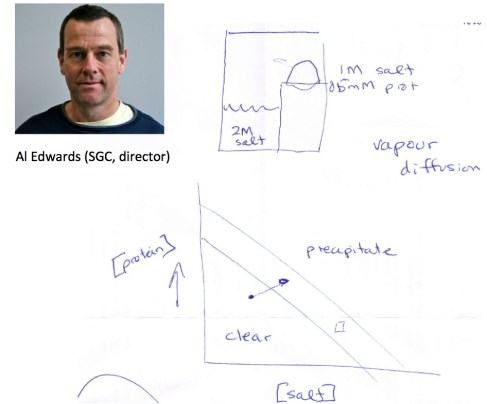
I am going to talk about some of my own work today. My sabbatical is going well, but I can sense that the Winter semester is around the corner. As of January 1 I am back to my regular job at the Chemistry Department. Thus far, my experience at SGC (http://www.sgc.utoronto.ca/sgc-webpages/sgc-toronto.php) has been a blessing. I have learned a lot of new things about structural biology. More importantly, some of my students are now trained in protein chemistry and crystallization (thanks to Elena). I also have to thank my good friend Al Edwards, who was at the beginning of it all. I refer to his role in the foundation of SGC together with Cheryl Arrowsmith as well as to his shaping of my interests in protein crystallography. Below is Al, by the way, along with a drawing he made at Starbucks some 6 years ago when we discussed standard conditions for crystal growth by slow diffusion. It all looks deceptively simple, but the devil is in the details. The trick is to get into the right “zone” that is conducive to crystal growth. Passing that zone is easy and it is all about conditions, conditions, and conditions (to paraphrase the real estate dictum).
As far as me doing lab work, here is the man lesson thus far: it is really good for an academic to go back and get calibrated in the lab from time to time. Besides its educational value, this experience reminds me about how rarely things actually work in research. Ironically, this is something that is special about research experience because we feel good when things do work. At the same time, if one’s heart is not 100% in research, failures can be a real drag. I have always enjoyed the challenge, so it is ok for me. But I will tell you this: if you are a professor and are sitting in your ivory tower overlooking a range of projects, it is easy to forget that each of your graduate students is in fact focusing on a specific and often narrow area of research. There is a significant ramification here: it is more difficult to get frustrated in the ivory tower. If there are no results in a particular project, there is always another, parallel, area that does generate results. In time, things fluctuate in terms of success between the areas. It certainly is tougher to be in the students’ shoes. It just ain’t the same when your job is fixed to one problem. Now… there is a practical and obvious lesson here for the students: always (yes, always) balance several different projects on your plate. In this case, the chance of getting discouraged about lack of results will be smaller.
For example, we evaluated a battery of new conditions aimed at understanding macrocycles and their folding in the crystalline state. I hope we’ll get some new hits but things have been difficult thus far. I set up 480 experiments and one of them worked, giving me crystals that unfortunately did not diffract today. But that’s ok – a couple of hours later I went back to my chemistry lab and talked to Piera, Adam, Shinya, Frank, and Victoria about their plans in boron chemistry. See: there was a total “switch”. We failed with our crystals earlier in the day, yet I got rejuvenated after a switch to something totally different. We bounced ideas and things are looking up… It’s all about balance.
On our future and methanol economy
Today I learned that my good friends and former mentors (can they ever be “former”, though?), Professors Prakash and Olah of USC, jointly received Israel’s 2013 Eric and Sheila Samson Prime Minister’s Prize for Innovation in Alternative Fuels for Transportation for their work in the area of methanol economy (http://www.latimes.com/world/worldnow/la-fg-wn-usc-professors-win-1-million-israeli-prize-for-energy-research-20131016,0,803168.story). Some words about the concept of methanol economy are in order. As Prakash likes to point out, despite the hysteria in the mass media, there is no shortage of energy on earth. Indeed, we have way too many energy-rich covalent bonds on our planet. Rather, there is definitely a shortage of convenient CARRIERS of energy, which is why modern energy research is akin to a gold rush. There are many ideas bouncing around and there are people who support vastly different views on how we will all try to survive given the shortage of cheap carriers. Methanol economy is the concept that has been advanced by Olah and Prakash over the past 15 years. If I were asked to sum it up, I would show something like this:
In methanol economy, methanol would be generated from methane and would be used as either a commodity chemical to access materials (using a number of known processes) or as a fuel. Electrochemical oxidation of methanol would provide energy plus carbon dioxide and water as by-products. Carbon dioxide is the greenhouse gas and so is methane. However, carbon dioxide reduction would allow for “feedback” within the loop shown above, provided that conversion back to methanol is cost-efficient. Because the carbon dioxide “input” comes from a variety of sources, closing this loop to make methanol (the simplest “C1” fuel) is a very worthy goal.
As we all know, there are also proponents of hydrogen economy. I still remember our group meetings from the middle of 1990’s when Olah would make a very simple and convincing point that not too many people would be thrilled to ride on a tank of compressed hydrogen in their cars. Who knows, though, we will see how it all develops. I do like the methanol economy idea because of its simple C1 link to carbon dioxide, which is one of the culprits behind global warming.
With that I want to congratulate Prakash and Olah one more time for their relentless efforts to advance the idea of methanol economy, which was recognized earlier today. Not bad for the city of Los Angeles – a Nobel to Warshel last week and this recognition to Prakash and Olah! By the way, here is a great book that talks about the methanol economy concept at length:
Give ChemSpider a chance
We all want to use reactions that work. OrgSyn is a portal into that privileged class of processes because there are people who actually reproduce everything that is being submitted. With the continuing expansion of resources available on the internet, I started noticing a range of other mechanisms that involve feedback from the users. I have always thought that Wikipedia was a great idea that has been displacing conventional Encyclopedias (when was the last time you looked at anything in Encyclopedia Britannica, for instance? Exactly… You no longer do it…) because of one profound and useful concept: user involvement. User-corrected articles are the way to go and I am convinced that we will see more of this applied to science in the future. But how about the present? There are some really marvellous initiatives out there with ChemSpider Synthetic Pages being one of them. This is a U.K. Royal Society of Chemistry’s program which I learned about at the Editors’ meeting in Brussels last Spring. I love this engine. Please take a look at the link below. I highly recommend using it in order to scour for procedures and comments by the users:
And all of you grad students out there – why not try to publish some of your tricks there? This will be a neat addition to your resume that will show that you are “modern resource”-savvy.
From color purple to alkenes
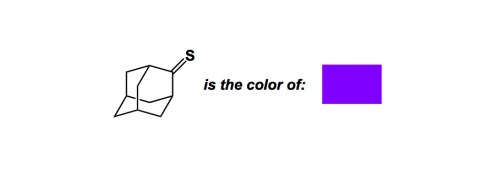
The color of adamantane thione is deep purple, which makes it one of the most strikingly beautiful compounds I have had a chance to observe (I also like the rock band…). The first time I saw adamantane thione was in 1995, when Dr. Grzegorz Mloston, a visiting scientist in the Olah lab, showed me a column onto which he loaded this compound. The band’s movement was sharp and purposeful, as if the molecule was trying to assert itself and tell me that color is not always due to the presence of some aromatic donor/acceptor chromophore. Indeed, I had to do some reading in order to understand why this color was so intensely bright!
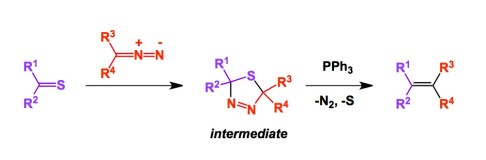
It was at about that time that I got interested in thiones. They do carry a couple of unfortunate “side effects”. First, they are not particularly stable to hydrolysis. This brings about the next challenge as these compounds are malodorous due to the hydrolyzed hydrogen sulfide, which our olfactory system can detect in exceedingly small amounts. Nonetheless, the relatively weak conjugation between carbonyl carbon and sulfur atoms enables some absolutely fascinating applications and I will talk about one of them: the Barton-Kellogg method. I saw it in action some 20 years ago and I have since considered the reaction to be a very special, albeit less widely utilized, method for making alkenes. We typically think of Wittig reaction, metathesis, eliminations, etc. However, if you want to make a “sterically challenged” alkene, few methods come close to the Barton-Kellogg process. During this reaction, a five-membered intermediate is created as a result of a dipolar cycloaddition. Triphenylphosphine then triggers the decomposition of the five-membered ring, liberating alkene, gaseous nitrogen, and sulfur.
Professor Huisgen, the father of dipolar cycloaddition reactions, is in such awe of thiones that he refers to them as superdipolarophiles. Take a look at the reference link below.
http://onlinelibrary.wiley.com/doi/10.1002/hc.20262/abstract
From chemical weapons to organic food…
Today I will lament on the choice of words when popular culture and the news media describe anything related to chemistry. It is well known that the corresponding outlets consistently represent chemistry in a very negative way. I am not talking about junk such as National Enquirer and the likes. Here is a high profile case: the Nobel Peace Prize, which was given today to the Organization for the Prohibition of Chemical Weapons “for its extensive efforts to eliminate chemical weapons”. I have always had a big problem with this wording “chemical weapons”. Are they chemical as opposed to all those other types of weapons that are composed of/made without chemicals? Show me those, please. I know, I know… I am being a little bit facetious since we all know what the intended meaning is. However, for many people the horrors of chemical weapons are merely associated with the word “chemical”, which carries a negative connotation in our society. It is clear that nerve gases have to be banned and destroyed. But I do point at the label “chemical” to show how science-illiterate our society has become. These sorts of blunders are ingrained in our language and have been with us for a long time. Dangerously, poor wording often allows people to knowingly misrepresent stuff. How about I give it a shot now?
Take the so-called organic food as an example. Out of sheer curiosity I once asked a worker at a local supermarket what an “organic” vegetable is meant to represent. Try it yourself. You will hear that “organic” represents a chemical-free way of growing vegetables. Wow… not even water (a chemical) for irrigation?
There is a silver lining here: to be an organic chemist makes me feel really good. From now on, I will tell people that in our lab we run chemistry without any chemicals. After all, if the moniker “organic” means what it is supposed to mean according to its (mis)use, then deal with it. Lastly, I really wonder how all those inorganic chemists look at themselves in the mirror each day. They are INorganic, after all (“IN” is a negative)… I guess whatever they do in their labs runs counter to the virtuous discoveries that we, organic folks, work so hard to make without any chemicals…
Hydrazine bonanza
I became aware of André Beauchemin’s really interesting Angewandte paper that appeared the other day. André’s lab at the University of Ottawa has been pushing the frontiers of synthesis with some imaginative use of heteroatom-heteroatom bonds as platforms for atom transfer chemistry. The Angewandte paper I refer to deals with novel amphoteric reagents, something that has been of particular interest to my lab for a number of years. Take a look at the hydrazine isocyanate André has developed:
http://onlinelibrary.wiley.com/doi/10.1002/anie.201306379/abstract
The isocyanate molecule shown above is a kinetically amphoteric reagent with the electrophilic carbonyl group of the isocyanate carbon placed several Angstroms away from its nucleophilic hydrazine nitrogen. Due to this constellation of reactive loci in the dense area of space, it is possible to add an amine nucleophile to the carbonyl carbon and quickly follow it up by ring formation “around the corner”. This is accomplished using Andre’s previously reported Cope-type chemistry. The resulting molecules are novel and interesting from the diversity/medicinal chemistry points of view. For me though, it is the amphoteric nature of the centerpiece that is of particular note.
Incidentally, back in 2006, when Ryan Hili and I were thinking about the best way to call our aziridine aldehyde dimers, we really liked the term “amphoteric” due to the origins of the word (“both of two” in Greek). We felt that naming the molecule “bifunctional” would not be descriptive enough. I am not a fan of the term “bifunctional” to begin with… What does it mean? I even get confused with “biweekly”: you think it is unambiguous, but some people use the word to refer to an event taking place once in two weeks, while others – two times a week. I think this is confusing…
2013 Nobel Prize in Chemistry
This one has been long overdue… As you all likely know, the 2013 Nobel Prize has been awarded to Karplus, Levitt, and Warshel for their ground-breaking theoretical work that has enabled everything from modelling the way enzymes carry out chemical transformations to investigating receptor/ligand interactions in drug design (http://www.nobelprize.org/nobel_prizes/chemistry/). Professor Ariel Warshel, whom I know personally (more on that soon), is well known for using a combination of quantum and classical mechanics to tackle the seemingly insurmountable challenges of complex protein simulation. Interestingly, and despite the nature of this work, one of Ariel’s points is that a great question is the most important thing in his branch of science. The computing power is secondary. He has maintained over the years that it is erroneous to assume that the amazing things possible now are there due to the emergence of really strong computers. In Ariel’s view, he was able to pose and answer profound questions 35 years ago as well as he is able to do so today. It is all about which questions you ask, I suppose.
In our lab, we feel the significance of the work credited with this year’s Nobel particularly strongly these days as we delve more and more into building a bridge between protein/small ligand crystallography and the design of chemical reactions that allow us to build better protein probes. Dr. Conor Scully, a research associate in our lab, has been the main driving force behind this project and the announcement of this year’s Nobel Prize really means a lot to all of us who use computers in efforts to understand chemistry.
I do have a bit of a personal connection with one of the winners (Ariel Warshel). When I came to the US in 1992, I had no training in synthesis. During my undergraduate years in Moscow I worked on developing an algorithm for enumerating reaction mechanisms with the help of graph theory (very obscure). So… I had a fellowship (which I declined) to go to Oxford and work with Prof. Graham Richards. I also had an offer from USC. Los Angeles sounded really exciting to me despite the 14 hour plane trip and the infamous Rodney King riots that burnt half of downtown in 1992 (my father said: “Where the hell are you going?”). But there I came. With my background, I started investigating the research advisors and Prof. Warshel was one of the people I spoke with at length. He is a real gentleman, originally from Israel, who was really nice to me, and it really meant a lot. However, synthetic and physical organic chemistry ultimately attracted me more and I joined the labs of Prakash and Olah. When I was watching the USC press conference dedicated to Warshel earlier today, Olah (the 1994 Nobel laureate) made a nice comment at the end, congratulating Ariel. He also said that they both arrived to USC at about the same time (end of 1970’s) and saw a significant transformation of the university. There was a cool moment during his commentary: Olah mentioned that football is great but it is research that should drive a university. This is one of his pet peeves and I fully agree with his stand. I am not sure you all know, but a football coach is typically the highest paid position at a major University in the US.
With this, I once again salute this year’s real heroes of science!