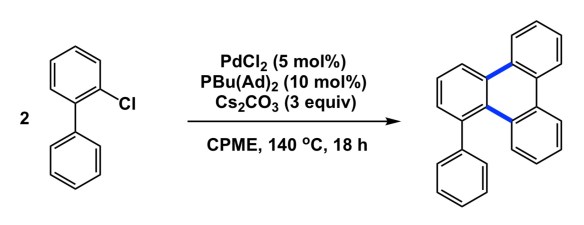
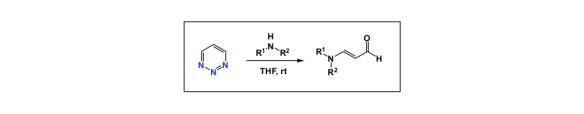
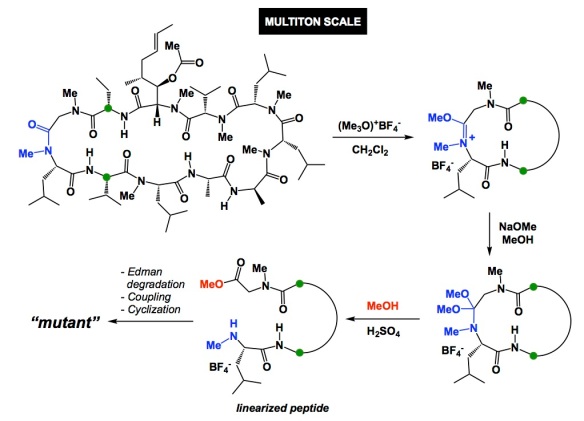
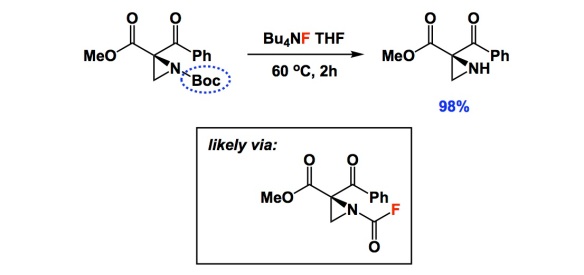
Have you ever noticed that, once a given idea gains acceptance in the community, everyone starts seeing its exemplification practically everywhere? I am not saying this is without merit, but I am sure you might agree that there was a time when no one looked at water as an “extra” in DFT calculations. Then water turned into an integral part of virtually every mechanism. Another example is when everyone started showing concerted metalation/deprotonation in C-H activation mechanisms. The list goes on and on.
One particular modern trend that I notice is towards involving single electron transfer in chemical transformations. What’s annoying is when people present this idea as an earth-shattering concept that is without precedent. I would respectfully remind everyone that there were several times in the past just like now. Electron transfer would become fashionable, and a ton of data would emerge to support its profound influence. Then people would forget it and move on, only to rediscover the virtues of moving electrons one at a time a decade or so later. This apparent amnesia gives me an opportunity to comment on an excellent paper from one of my heroes and mentors, the late Professor George A. Olah. Below is a fascinating piece from the ’80s, where Olah considered the generation of alcohols in the course of some Wittig reactions. Single electron transfer is the mechanism that accounts for the formation of the reduced product in this case. Yes, even the venerable Wittig reaction can involve electrons jumping one at a time!
https://pubs.acs.org/doi/abs/10.1021/ja00378a035?journalCode=jacsat